Impurity Reference Materials
Our quality impurity reference materials - including intermediates, by-products and degradation products - enable accuracy in both your qualitative and quantitative analysis, with the aim of helping you to create ever better, safer medicines.
Products in our Mikromol range of more than 4,400 pharmaceutical impurity reference materials each come with a comprehensive Certificate of Analysis detailing the material’s characterisation process, purity and traceability, in line with ICH Q3B and ICH Q7.
To complement our large portfolio of Mikromol impurity reference materials, we also offer a comprehensive selection of impurity analytical standards, covering more than 10,000 impurities, in our TRC range.

- Product Code: MM2115.00-0250
- CAS Number: 345-78-8
- Brand: Mikromol
- EP Description: Ephedrine Hydrochloride Impurity B as Hyd...
- Product type: Impurity, API
- Product Format: Neat
- API family: Ephedrine Hydrochloride, Pseudoephedrine ...


- Product Code: MM0431.00-0250
- CAS Number: 99-76-3
- Brand: Mikromol
- EP Description: Butyl Parahydroxybenzoate Impurity B, Et...
- Product type: Impurity, Excipient, API
- Product Format: Neat
- API family: Methyl Salicylate, Methyl Parahydroxybenz...

- Product Code: MM0502.00-0250
- CAS Number: 16051-77-7
- Brand: Mikromol
- EP Description: Isosorbide Dinitrate Impurity C
- Product type: Impurity, API
- Product Format: Neat
- API family: Isosorbide Dinitrate, Diluted, Isosorbide...

- Product Code: MM0045.00-0250
- CAS Number: 69-72-7
- Brand: Mikromol
- EP Description: Acetylsalicylic Acid Impurity C, Carbasa...
- Product type: Impurity, Excipient, API
- Product Format: Neat
- API family: Acetylsalicylic Acid, Salicylic Acid, Hyd...

- Product Code: MM0089.00-0250
- CAS Number: 1218-35-5
- Brand: Mikromol
- EP Description: Oxymetazoline Hydrochloride Impurity B as...
- Product type: Impurity, API
- Product Format: Neat
- API family: Oxymetazoline Hydrochloride, Xylometazoli...
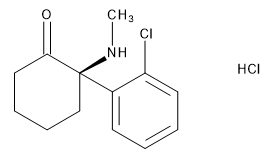
- Product Code: MM0144.10-0025
- CAS Number: 33795-24-3
- Brand: Mikromol
- EP Description: Esketamine Hydrochloride Impurity D as Hy...
- Product type: Impurity, API
- Product Format: Neat
- API family: Esketamine Hydrochloride, Ketamine Hydroc...

- Product Code: MM0045.01-0250
- CAS Number: 99-96-7
- Brand: Mikromol
- EP Description: Acetylsalicylic Acid Impurity A, Butyl P...
- USP Description: Salicylic Acid Related Compound A
- Product type: Impurity, API
- Product Format: Neat
- API family: Acetylsalicylic Acid, Salicylic Acid, Met...
- 1
- 2
- 3
- 4
- 826










