Excipient Reference Materials
Excipients are inactive ingredients formulated alongside an Active Pharmaceutical Ingredient (API) for purposes such as stability and therapeutic enhancement - including facilitating drug absorption or enhancing drug solubility.
Recent studies have demonstrated that excipients can also play a role in the introduction of potentially hazardous impurities - such as small molecule nitrosamines - into pharmaceutical products, meaning that they must be comprehensively risk assessed and carefully monitored throughout the drug development process.
A qualified, GMP/GDP compliant supplier with EXCiPACT certification, we offer more than 180 excipient reference materials manufactured according to ISO/IEC 17025 requirements, and an increasing number accredited to ISO 17034. Suitable for use with non-compendial analytical methods, each comes with a detailed Certificate of Analysis (CoA) describing its characterisation.
Recent studies have demonstrated that excipients can also play a role in the introduction of potentially hazardous impurities - such as small molecule nitrosamines - into pharmaceutical products, meaning that they must be comprehensively risk assessed and carefully monitored throughout the drug development process.
A qualified, GMP/GDP compliant supplier with EXCiPACT certification, we offer more than 180 excipient reference materials manufactured according to ISO/IEC 17025 requirements, and an increasing number accredited to ISO 17034. Suitable for use with non-compendial analytical methods, each comes with a detailed Certificate of Analysis (CoA) describing its characterisation.
Filter results
Promotion
API Family
Refine or expand your search with AND/OR
OR
Select or search...
Product Type
OR
Brand
Product Accreditations
Lab Accreditations
Analyte
CAS Number
Product Format
Impurity Type
SIL Type
48-hour USA dispatch time

- Product Code: MM0431.00-0250
- CAS Number: 99-76-3
- Brand: Mikromol
- EP Description: Butyl Parahydroxybenzoate Impurity B, Et...
- Product type: Impurity, Excipient, API
- Product Format: Neat
- API family: Methyl Salicylate, Methyl Parahydroxybenz...
ISO 17034

- Product Code: MM0102.04-0250
- CAS Number: 6108-05-0
- Brand: Mikromol
- Product type: Impurity, Excipient, API
- Product Format: Neat
- API family: Lidocaine Hydrochloride Monohydrate
ISO 17034

- Product Code: MM0045.00-0250
- CAS Number: 69-72-7
- Brand: Mikromol
- EP Description: Acetylsalicylic Acid Impurity C, Carbasa...
- Product type: Impurity, Excipient, API
- Product Format: Neat
- API family: Acetylsalicylic Acid, Hydroxyethyl Salicy...
ISO 17034

- Product Code: MM0167.00-0250
- CAS Number: 10191-41-0
- Brand: Mikromol
- EP Description: all-rac-a-Tocopheryl Acetate Impurity C
- Product type: Impurity, Excipient, API
- Product Format: Neat
- API family: all-rac-alpha-Tocopherol, Tocopheryl Acet...
ISO 17034
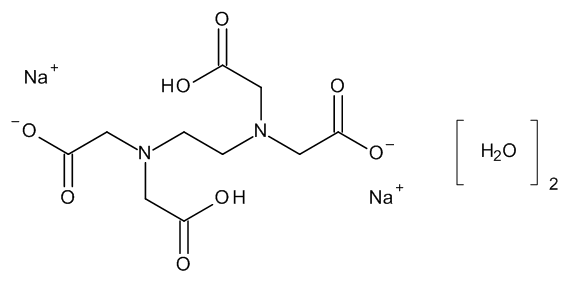
- Product Code: MM0886.00-0250
- CAS Number: 6381-92-6
- Brand: Mikromol
- Product type: Impurity, Excipient, API
- Product Format: Neat
- API family: Disodium Edetate
ISO 17034

- Product Code: MM0432.00-0250
- CAS Number: 94-13-3
- Brand: Mikromol
- EP Description: Butyl Parahydroxybenzoate Impurity D, Et...
- Product type: Impurity, Excipient, API
- Product Format: Neat
- API family: Methyl Parahydroxybenzoate, Propyl Parahy...
ISO 17034

- Product Code: MM0278.01-0250
- CAS Number: 65-85-0
- Brand: Mikromol
- EP Description: Benfluorex Hydrochloride Impurity C, Ben...
- Product type: Impurity, Excipient, API
- Product Format: Neat
- API family: Benzoyl Peroxide Hydrous, Benfluorex Hydr...
ISO 17034

- Product Code: MM0494.00-0250
- CAS Number: 58-08-2
- Brand: Mikromol
- EP Description: Dimenhydrinate Impurity C, Pentoxifyllin...
- Product type: Impurity, Excipient, API
- Product Format: Neat
- API family: Caffeine Citrate, Theophylline-Ethylenedi...
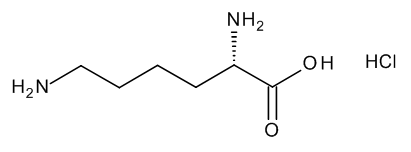
- Product Code: MM1521.00-0250
- CAS Number: 657-27-2
- Brand: Mikromol
- Product type: Impurity, API
- Product Format: Neat
- API family: Lisdexamfetamine Dimesilate, Lysine Hydro...
ISO 17034
- 1
- 2
- 3
- 4
- 18