Pharmaceutical roots is a new content series from LGC Mikromol, investigating and outlining the natural origins of pharmaceutical substances, and offering a deeper dive into their uses, risks, and mechanisms of action.

Morphine is a type of opiate pain medication with a strong analgesic duration, lasting from three to seven hours. Named after Morpheus, the Greek god of dreams, morphine is generally believed to be the first active ingredient ever to be isolated from a plant, having been first isolated in the early 19th century. It has since played a vital role in the history of chemical neuroscience ever since.
Morphine belongs to a class of chemical compounds called alkaloids. Many organisms produce these basic organic compounds, which contain at least one nitrogen atom. What makes morphine particularly interesting is that it is only produced in significant amounts by one plant – Papaver somniferum, or the Opium Poppy.
The history of opium
The opium poppy is a member of Papaveraceae, a large family comprising over 700 species of flowering plants. The family is distributed over a wide range of climates primarily in the Northern Hemisphere, with tribes occurring in North America, Eastern Mexico, Europe and Asia.
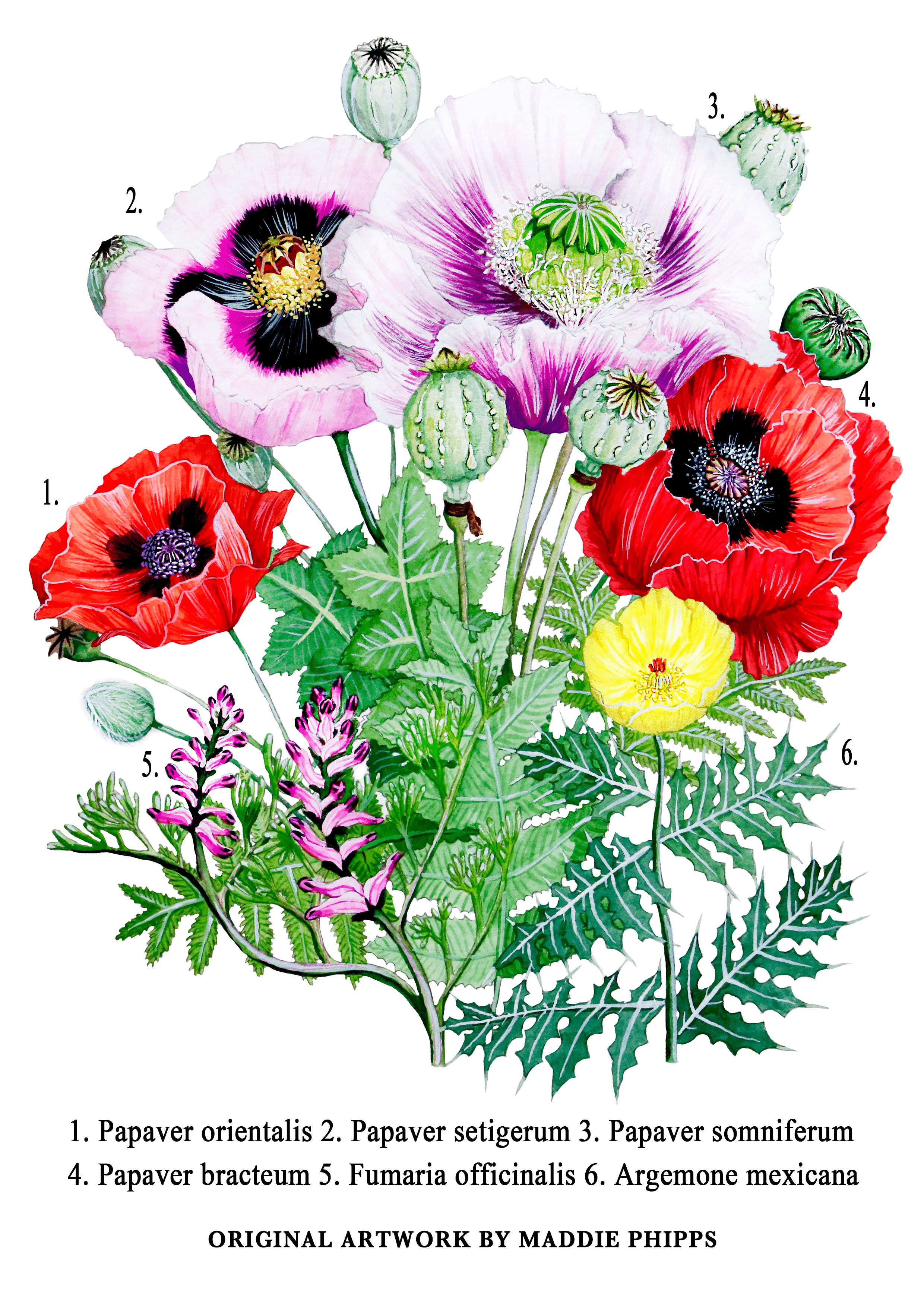
The plants vary wildly from flower to flower, but all have in common the production of a milky substance designed to protect the plant from herbivores known as latex. The latex of Papaveraceae have a rich phytochemistry ranging from fumaric acid, a fruity-tasting food additive found in the latex of Fumaria officinalis, to the toxic alkaloid sanguinarine, produced by Argemone Mexicana, Mexican prickly poppy. When dried, the milky-white latex produced by P. somniferum turns into a sticky, brown resin, which we know as opium.
Opium can be smoked, eaten, or drunk, and has been said to “banish fatigue and pain, to stimulate the mind and liberate the user from nervousness or worry”. These feelings of euphoria and lessening pain are largely due to morphine, which accounts for around 10% of the total alkaloid content of opium.
Evidence of humans using opium dates back over 5000 years. Poppy capsules have been discovered in a small Neolithic village in Switzerland. Sumerians referred to the poppies as a “joy plant”, whilst the ancient Greeks associated poppies with the gods and goddesses. Homer also wrote about opium potions in his poems, and Hippocrates recommended poppy juice for many different ailments. However, it was not until the 16th century that opium was used medicinally in the Western world. Paracelsus, a Swiss-German alchemist, promoted the use of laudanum (from laudere, “to praise”), an extract of opium mixed with alcohol, for many ailments with the exception of leprosy. Many variations of laudanum followed, and the concoctions were available without prescription until the early 20th century.
Isolation of morphine and other alkaloids
In 1803, the German pharmacist Friedrich Sertürner began experimenting with opium, and in 1806 succeeded in the isolation of morphine. This was the first alkaloid ever extracted from both opium and plants in general. Morphine was used as an analgesic in the 1830s and as a rapid analgesic in 1853 following the invention of the hypodermic needle. Interestingly, it was thought that addiction processes occurred in the stomach, and that one would not become addicted to morphine if it were injected.
Other alkaloids present in large amounts in opium are papaverine, noscapine, codeine and thebaine. Papaverine was discovered in 1848 by Georg Merck and is used to treat erectile dysfunction. Noscapine was first isolated in 1817 and sometimes used as a cough suppressant. Papaverine and noscapine have very different structures from morphine, codeine, and thebaine, and are neither psychoactive nor addictive.
The French chemist Pierre Jean Robquet discovered codeine in 1832 – he was also the isolator of noscapine. Codeine is less addictive and psychoactive than morphine or thebaine and is often used as a cough suppressant, or in conjunction with paracetamol as a painkiller. P. orientalis and P. bracteatum also produce thebaine, an important raw material for many opioids. Thebaine derives its name from the Ancient Egyptians, who restricted opium use to priests, magicians, and warriors – opium-based medications were, for a time, called thebaics, after the River Thebes.

The terms ‘opiate’ and ‘opioid’ are often used interchangeably, but they have different meanings. Opiates are the natural alkaloids obtained from the opium poppy. Unfortunately, many of these are highly addictive.
Extensive research into semi-synthetic or synthetic opioids has been carried out, with hopes of finding a less addictive compound. As such, the opium family is a large one with semisynthetic opiate derivatives including oxycodone (from thebaine), hydrocodone (from codeine), and hydromorphone (from morphine) alongside opioid antagonists such as naloxone and naltrexone (both thebaine derivatives). There also exists a large number of synthetic opioids (almost 150) such as pethidine, fentanyl and methadone. Many of these compounds are less addictive but also less effective as analgesics, such as the cough suppressant pholcodine. Others, such as etorphine, are so potent they can be used to tranquilise elephants and walruses.
Perhaps the most infamous opioid is, of course, diacetylmorphine. In 1874, whilst on the hunt for a non-addictive morphine, English chemist Charles Wright boiled morphine and acetic anhydride, yielding diacetylmorphine. Wright carried out no further research on this compound and it was over 20 years later, in 1897, that German researcher Felix Hoffman re-discovered the acetylated substance. Bayer began marketing this new drug, calling it “Heroin” (based on the German “heroisch”, meaning heroic), as a non-addictive morphine-alternative cough suppressant. Of course, it was soon realised that heroin was twice as potent as morphine with extremely high addiction rates. In 1924, the United States Congress banned its sale and production.
Looking forwards
Although total synthesis of morphine has been realised, methods are not efficient enough to meet global demand. Opium poppies are still cultivated around the world – Turkey, Australia and India are amongst the leading contributors for licit opium poppies for medicinal products. India and Turkey used to contribute to illegal poppy cultivation, but had the status of their country changed due to disagreement of countries struggling with issues of heroin addiction, such as the United States. Today, countries including Afghanistan, Guatemala, and Mexico are responsible for the majority of illicit opium poppy cultivation for heroin production.
Currently there is ongoing research into new, potentially more efficient methods of obtaining opiates, by way of biosynthesis. For over ten years, researchers in Montreal, Canada, have been attempting the genetic modification of yeast to synthesise bioactive molecules. By reconstructing plant pathways mediating synthesis morphine precursor (S)-reticuline, they have been able to increase the amount of precursor produce (to 4.6 gL-1) and provide a platform for morphine synthesis. In other studies in Japan, researchers have manipulated strains of Escherichia coli to synthesise thebaine from increased production of (R)-reticuline. Both groups have the aim of scaling the these platforms up to industrial level.
About the author

Dulcie Phipps is an Assistant Global Product Manager at LGC. A chemist with a passion for nature, she grew up in the glens of Perthshire, Scotland.
Phipps’ academic background includes a Marine Science HNC, an MSc in Chemistry from the University of Glasgow, and a European work placement focusing on lanthanide chemistry at Uppsala University, Sweden. After completing her degree she moved to Luckenwalde, Germany, to work for LGC.
