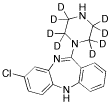
N-Desmethyl Clozapine
| ||||
Product Code
TRC-D292000
CAS Number
EP Description
Clozapine Impurity C
USP Description
Clozapine Impurity C
Product Format
Neat
Molecular Formula
C17 H17 Cl N4
Molecular Weight
312.80
API Family
ClozapineProduct Categories
TRC, Antipsychotics, Benzodiazepines Reference Materials , Agonist & antagonist analogues, Impurity Reference Materials , Acetylcholine Receptors, Neurotransmission, Memory, Learning and Cognition, Parkinson's, Schizophrenia, Pain and Inflammation
Product Type
MetabolitePurity
>95% (HPLC)
Related Products
Documentation
Product Information
Chemical Data
Product Data
Product Description
{{title}}
Please login or register to add to your favourites
Or continue browsing without access to favourites or pricing
Please log in to view pricing and add to cart
Or continue browsing to see available rounds without pricing information
If you don't yet have an account, please create an account create an account
- Product Code:
{{ isMultiplePacksize(product) ? getSourcePart(product) : product.code }} {{ isMultiplePacksize(product) ? getSourcePart(product) : product.code }}
Controlled product
Login to view full product details
{{ addToCartData.mixPtRmWarning }}
Do you want to proceed?
{{ errors.first('requestQuoteForm.firstName') }}
{{ errors.first('requestQuoteForm.lastName') }}
{{ errors.first('requestQuoteForm.email') }}
{{ errors.first('requestQuoteForm.phoneCountryCode') }}
{{ errors.first('requestQuoteForm.phoneNumber') }}
{{ errors.first('requestQuoteForm.company') }}
{{ errors.first('requestQuoteForm.selectedShippingAddress') }}
{{ errors.first('requestQuoteForm.shippingCompanyName') }}
{{ errors.first('requestQuoteForm.shippingLine1') }}
{{ errors.first('requestQuoteForm.shippingCountry') }}
{{ errors.first('requestQuoteForm.shippingState') }}
{{ errors.first('requestQuoteForm.shippingCity') }}
{{ errors.first('requestQuoteForm.shippingPostCode') }}
{{ errors.first('requestQuoteForm.billingCompanyName') }}
{{ errors.first('requestQuoteForm.billingLine1') }}
{{ errors.first('requestQuoteForm.billingCountry') }}
{{ errors.first('requestQuoteForm.billingState') }}
{{ errors.first('requestQuoteForm.billingCity') }}
{{ errors.first('requestQuoteForm.billingPostCode') }}
{{ errors.first('requestQuoteForm.deliveryCountry') }}
{{ errors.first('requestQuoteForm.packSize') }}
{{ errors.first('requestQuoteForm.quantity') }}