Mikromol featured product: Diclofenac
Featured product
Diclofenac
API and impurity reference standards
Introduction
Diclofenac (sold under the brand names Voltaren, Solaraze, Cambia and Lofena, among many others) is a non-steroidal anti-inflammatory drug (NSAID) with analgesic, antipyretic and anti-inflammatory properties. It is commercially available as tablets, capsules, dragees, suppositories, gels, patches, eye drops, and as a solution of injection. Diclofenac is used in the management of pain, various inflammatory conditions , and dysmenorrhea (menstrual cramps).
Diclofenac was patented in 1965, and first introduced in Switzerland in 1973, before coming into medical use in the United States in 1988. Also available as a generic medication, it was the US’s 72nd most commonly prescribed medicine in 2020, with over nine million prescriptions. Diclofenac sodium was initially developed with the goal of improving the safety profile of diclofenac and providing convenient, once-daily dosing for the treatment of chronic pain. New drug products, using diclofenac potassium salt, are associated with faster absorption and rapid pain relief. These include diclofenac potassium immediate-release tablets, liquid-filled soft gel capsules, and powder for oral solution.
Mechanism of action
Diclofenac acts through the inhibition of the enzyme cyclooxygenase (COX-1 and -2) and the reduced synthesis of prostaglandins (PGs). PGs have broad activity in inflammation and pain signalling, and are involved in modulation of nociception – mediating acute inflammation via their IP and EP2 receptors. By limiting the production of these PGs at the site of injury, diclofenac can reduce inflammation and pain. In addition, PGE2 can cross the blood-brain barrier and act on receptors on thermoregulatory neurons - triggering an increase in heat generation and reduction in heat loss, and resulting in a fever. By inhibiting PGE2 generation, diclofenac can reduce the activity of these neurons. Diclofenac has a short half-life of one to three hours and is therefore also administered in sustained-release forms.
Why not browse our range of diclofenac API and impurity reference standards below?
Diclofenac Sodium
| Imp. A (EP): 1-(2,6-Dichlorophenyl)-1,3-dihydro-2H-indol-2-one
| Imp. B (EP): 2-[(2,6-Dichlorophenyl)amino]benzaldehyde
|
Imp. C (EP): [2-[(2,6-Dichlorophenyl)amino]phenyl]methanol
| Imp. D (EP): 2-[2-[(2-Bromo-6-chlorophenyl)amino]phenyl]acetic Acid
| Imp. D (EP) as Sodium Salt: Sodium 2-[2-[(2-Bromo-6-chlorophenyl)amino]phenyl]acetate
|
Imp. E (EP): 1,3-Dihydro-2H-indol-2-one
| Imp. F (EP): N-(4-Chlorophenyl)-2-(2,6-dichlorophenyl)acetamide
| 1-(2-Bromo-6-chlorophenyl)indolin-2-one
MM0006.06 & MM0006.06-0025
|
Methyl 2-[2-[(2,6-Dichlorophenyl)amino]phenyl]acetate (Methyl Ester of Diclofenac)
| Ethyl 2-[2-[(2,6-Dichlorophenyl)amino]phenyl]acetate (Ethyl Ester of Diclofenac)
| 2-(2,6-Dichlorophenylamino)benzoic Acid
|
2,6-Dichlorodiphenylamine
| 2-Bromo-6-chlorodiphenylamine
| 1-(2,6-Dichlorophenyl)indolin-2,3-dione
|
Diclofenac Potassium
| 2-Chloro-N-(2,6-dichlorophenyl)-N-phenylacetamide
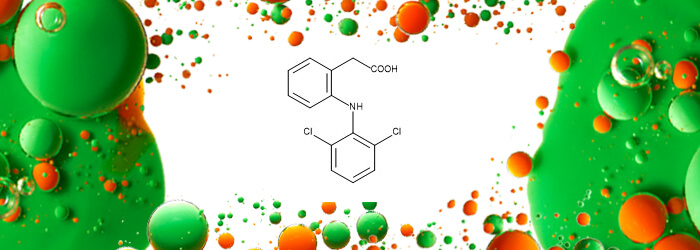
| 2-[2-[(2,6-Dichlorophenyl)amino]phenyl]acetic Acid (Diclofenac)
|
2,6-Dichloroaniline
| 2-Bromo-6-chloroaniline
| 4'-Hydroxydiclofenac
| |
Dimethylammonium Diclofenac
| N-Acetyl-N-phenyl-2,6-dichloroaniline
| Diethyl Phthalate
| |
2'-Chloroacetanilide
| Bromobenzene
| Diclofenac 2,3-Butylene Glycol Ester
| |
Diclofenac 1,2-Propylene Glycol Ester (Mixture of Isomers)
| Diclofenac Butylene Glycol Ester (Mixture of Isomers)
| ||


+1-(2%2C6-Dichlorophenyl)-1%2C3-dihydro-2H-indol-2-one+.jpg)
![Imp. B (EP) 2-[(2,6-Dichlorophenyl)amino]benzaldehyde](https://lgcstandards-assets.s3.eu-west-1.amazonaws.com/MediaGallery/Blog/Imp.+B+(EP)+2-%5B(2%2C6-Dichlorophenyl)amino%5Dbenzaldehyde.jpg)
![Imp. C (EP) [2-[(2,6-Dichlorophenyl)amino]phenyl]methanol](https://lgcstandards-assets.s3.eu-west-1.amazonaws.com/MediaGallery/Blog/Imp.+C+(EP)+%5B2-%5B(2%2C6-Dichlorophenyl)amino%5Dphenyl%5Dmethanol.jpg)
![Imp. D (EP) 2-[2-[(2-Bromo-6-chlorophenyl)amino]phenyl]acetic Acid](https://lgcstandards-assets.s3.eu-west-1.amazonaws.com/MediaGallery/Blog/Imp.+D+(EP)+2-%5B2-%5B(2-Bromo-6-chlorophenyl)amino%5Dphenyl%5Dacetic+Acid.jpg)
![Imp. D (EP) as Sodium Salt Sodium 2-[2-[(2-Bromo-6-chlorophenyl)amino]phenyl]acetate](https://lgcstandards-assets.s3.eu-west-1.amazonaws.com/MediaGallery/Blog/Imp.+D+(EP)+as+Sodium+Salt+Sodium+2-%5B2-%5B(2-Bromo-6-chlorophenyl)amino%5Dphenyl%5Dacetate.jpg)
+1%2C3-Dihydro-2H-indol-2-one.jpg)
+N-(4-Chlorophenyl)-2-(2%2C6-dichlorophenyl)acetamide.jpg)
indolin-2-one.jpg)
![Methyl 2-[2-[(2,6-Dichlorophenyl)amino]phenyl]acetate (Methyl Ester of Diclofenac)](https://lgcstandards-assets.s3.eu-west-1.amazonaws.com/MediaGallery/Blog/Methyl+2-%5B2-%5B(2%2C6-Dichlorophenyl)amino%5Dphenyl%5Dacetate+(Methyl+Ester+of+Diclofenac).jpg)
![Ethyl 2-[2-[(2,6-Dichlorophenyl)amino]phenyl]acetate (Ethyl Ester of Diclofenac)](https://lgcstandards-assets.s3.eu-west-1.amazonaws.com/MediaGallery/Blog/Ethyl+2-%5B2-%5B(2%2C6-Dichlorophenyl)amino%5Dphenyl%5Dacetate+(Ethyl+Ester+of+Diclofenac).jpg)
benzoic+Acid.jpg)


indolin-2%2C3-dione.jpg)

-N-phenylacetamide.jpg)
![2-[2-[(2,6-Dichlorophenyl)amino]phenyl]acetic Acid (Diclofenac)](https://lgcstandards-assets.s3.eu-west-1.amazonaws.com/MediaGallery/Blog/2-%5B2-%5B(2%2C6-Dichlorophenyl)amino%5Dphenyl%5Dacetic+Acid+(Diclofenac).jpg)









.jpg)
.jpg)